
IKNL
Prof. dr. A. Jager (EMC)
Dr. C. van den Hurk (Santeon)
Dr. C.H.C. Drossaert (UT)
Dr. ir. M. Mulder (EMC)
Clinical Study Manager: Susan van den Berg (BOOG SC)
Project assistant: Suzanne Jager (BOOG SC)
info@boogstudycenter.nl
Project leader / Principal investigator:
Prof. dr. Sabine Siesling (IKNL / UT)
Coordinating investigators:
Lois Folkert, MSc (IKNL / UT)
Lars Woudstra, MSc (IKNL)
Dr. Jolanda van Hoeve (IKNL)
sympha@iknl.nl
IKNL / Castor EDC
IKNL
Not applicable
KWF
Studieinformatie voor patiënten (run-in fase): https://www.profielstudie.nl/sympha-nulmeting/
Studieinformatie voor patiënten (RCT fase): https://www.profielstudie.nl/sympha/
Handige tips en uitlegvideo’s voor patiënten (over de BijKanker app en installatie van de Fitbit): https://www.profielstudie.nl/sympha-instructies/
Studieinformatie kanker.nl: https://www.kanker.nl/trials/1555-sympha-studie-borstkanker
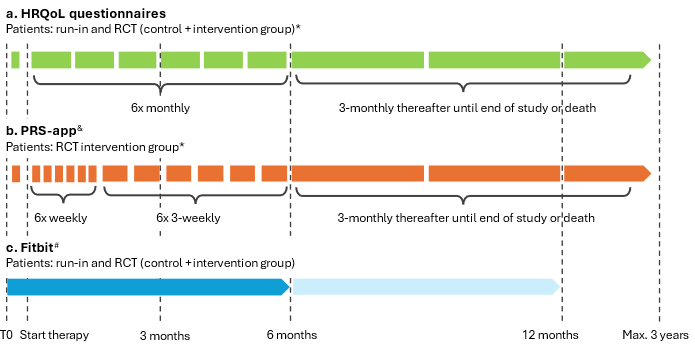
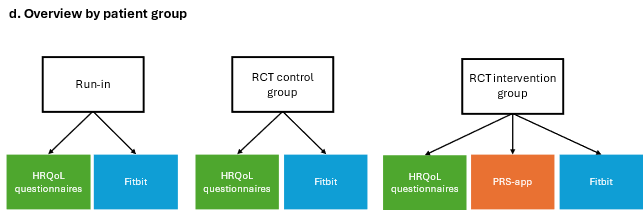
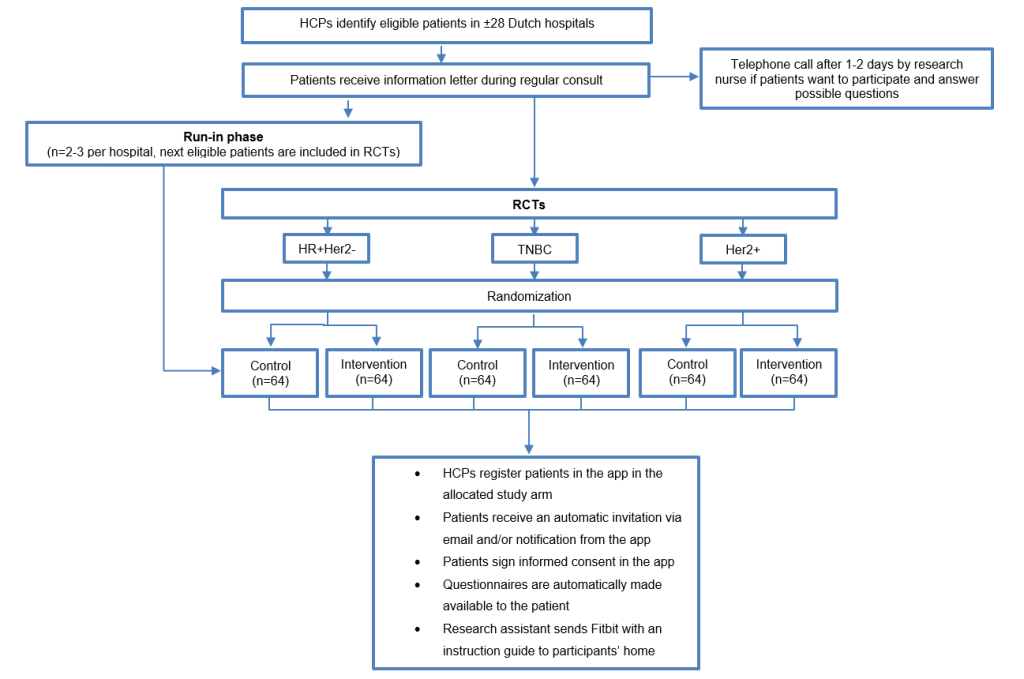
Basket trial: three separate clinical prospective open label 1:1 randomized clinical trials (RCTs) within the three different mBC subtypes (TNBC, HR+Her2- or Her2+) preceded by a run-in phase.
Primary objective
To determine the effect of real-time PRS monitoring on HRQoL (summary score) in patients with mBC who start first-line chemo(immune)therapy .
Secondary objectives
Primary endpoint
The main endpoint is the difference of HRQoL between the control and intervention arms on the mean difference between T0 (start of the first line systemic chemo(immune)therapy for mBC) and the mean HRQoL during the first line of therapy until first switch of therapy or, in case of no switch, six months.
Secondary endpoints
Secondary endpoints include differences between the control and intervention arms in the severity of symptoms, treatment patterns, therapy adherence, healthcare resource use, cost-effectiveness, long-term HRQoL, overall survival and patient empowerment. In addition, we strive for a first step towards a trained and (internally) validated model for early detection and prediction of symptoms through Fitbit data.
Inclusion criteria
For the Fitbit a mobile phone with internet and Bluetooth is required. Patients who are not able or not willing to wear the Fitbit can still participate in this trial and will only use the app.
Exclusion criteria