BOOG Study Center
Prof.dr. S.C. Linn, drs. H.M. Oosterkamp en dr. M. Kok
Dr. A.E. van Leeuwen-Stok, BOOG Study Center
Rosie Voorthuis, MD
Phone 020 – 512 7951
E-mail r.voorthuis@nki.nl
tripleB@nki.nl
Contact gegevens centraal datamanagement TRIPLE-B:
b.dufourny@nki.nl, tel 020 512 9046
Randomization:
NKI Data Center, Trial Office Phone +31 20 512 2668
Fax +31 20 512 2679, E-mail trial@nki.nl
Central contact NKI Data Center:
I. Mandjes Phone +31 20 512 2880, E-mail i.mandjes@nki.nl
NKI Data Center:
Karin Kaptijn
Phone +31 20 512 2655
E-mail k.kaptijn@nki.nl
IKNL / centrum zelf
Roche grant
Immunohub: NKI-AvL
Contact: tripleB@nki.nl
Primary:
Secondary:
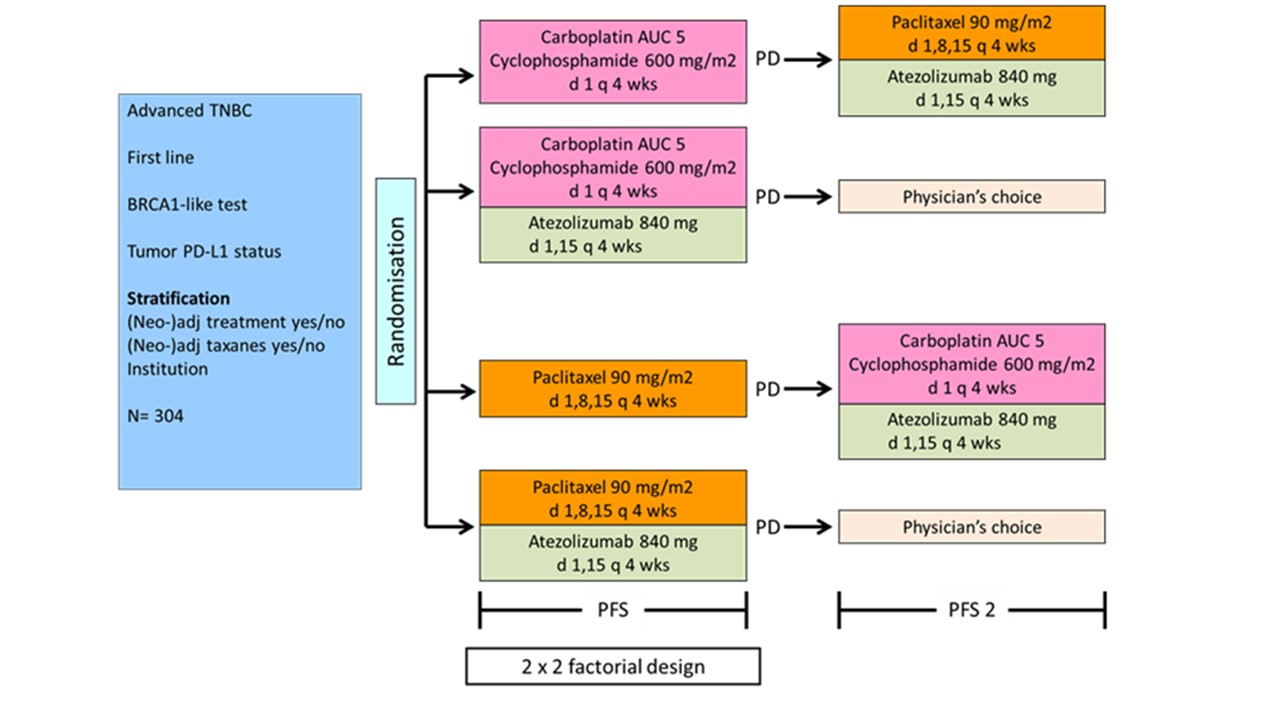
* BRCA-like status determined by array comparative genomic hybridization (aCGH), multiplex ligase-dependent probe amplification (MLPA), or next generation sequencing (NGS); BRCA-like test positive when the genomic profile resembles that of BRCA-mutated breast cancers (BRCA-like genomic profile, derived from BRCA1 or BRCA2 mutation), or when the tumor is BRCA1-mutated, BRCA2-mutated, the patient is a BRCA1 and/or BRCA2 germline mutation carrier, or has a BRCA1 promoter hypermethylation.
Primary endpoint:
Primary Outcome Measure (2×2 factorial design):
Secondary Outcome Measures: